Видео ютуба по тегу How To Keep A Solution At A Constant Ph
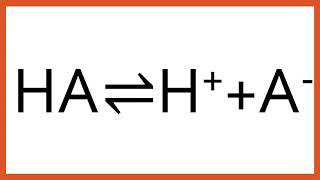
Buffer Solutions Explained Simply: What is a Buffer and How Does a Buffer Solution Work?

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems

Buffer Solutions

Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLE

Acid-Base Equilibria and Buffer Solutions

pH of Weak Acids and Bases - Percent Ionization - Ka & Kb

Buffer solution pH calculations | Chemistry | Khan Academy

AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables

pH and pKa relationship for buffers | Chemistry | Khan Academy

Buffer Solutions

pH and pOH: Crash Course Chemistry #30

Titration |#acidbase |#shorts |#ytshorts |#shortsfeed |#youtubevideos

Acid Dissociation Constant, Ka and pKa (A-Level Chemistry)

ALEKS: Calculating the Ka of a weak acid from pH

Ka from pH and a concentration
![How to Calculate pH of Weak Acids & Bases using ICE Table? [Singapore GCE A Level H2 Chemistry]](https://ricktube.ru/thumbnail/DfJt5ZbE-7c/mqdefault.jpg)
How to Calculate pH of Weak Acids & Bases using ICE Table? [Singapore GCE A Level H2 Chemistry]

CH15Q1 Identifying a buffer solution

R3.1.14 / R3.1.15 Acid-base indicators (HL)

Acid/Base Dissociation Constant

Calculate Concentration and pH ICE Table